Patient characteristics
A 68-year-old male requested a consultation after symptoms interfered with his work and daily activities. His symptoms included bilateral tortuous varicose veins that were tender to the touch, ankle swelling, skin discoloration, leg pain, and restless legs. His job as a roofer required him to be standing and sitting for prolonged periods of time leading to worsening of symptoms.
Previous intervention included two years of compression therapy that had unsuccessfully treated his symptoms and previous ablation procedures on both proximal GSVs.
Patient work-up: CEAP class 4a
- Duplex ultra sound reveled bilateral reflux in distal GSV sand tributaries. Skin changes were also present making the patient CEAP class 4.
- Right leg vein diameters were 3.0mm in the distal GSV and ranged from 3.1mm to 3.7mm in the calf tributaries.
- Left leg vein diameters were 4.7mm in the distal GSV, 5.3mm in the thigh tributaries, 4.4mm in the knee tributaries, and 4.2mm in the calf tributaries.
Treatment and results
It was determined that two sessions were needed, separated by one week, with the left leg undergoing treatment first.
In both procedures, the treated leg was mapped with ultrasound and access points were marked. A 25g butterfly needle was used for access, then the leg was elevated to 45 degrees.
The left leg was treated with 15cc of Varithena in two access sites (one proximal GSV site near the medial knee and one distal GSV site in the medial calf).
The right leg was treated with 13cc of Varithena in two access sites (one proximal GSV site near the lateral knee and one distal GSV site in the posterior calf).
After each procedure,the treated veins were observed for spasm, then compression wrapping and 20-30mmHg compression stockings were applied. The total treatment volume was 28cc of Varithena.
The patient followed up in clinic one month post procedure. He had significant symptom improvement and was able to return to work. He said he was able to go up and down ladders without any pain and stated his legs now feel wonderful.
Follow-up exams did not show any signs of superficial reflux or DVTs and treated veins were confirmed to be closed.
Conclusion
Due to this patients extensive bilateral disease, it was necessary to stagger his treatments over two visits. The procedure was tolerated well with no complications. Post-treatment instructions were followed and the patient stated he was very pleased with the results of the procedure.
The patient will follow-up in clinic for treatment of varicose tributaries if they become symptomatic.
Documentation

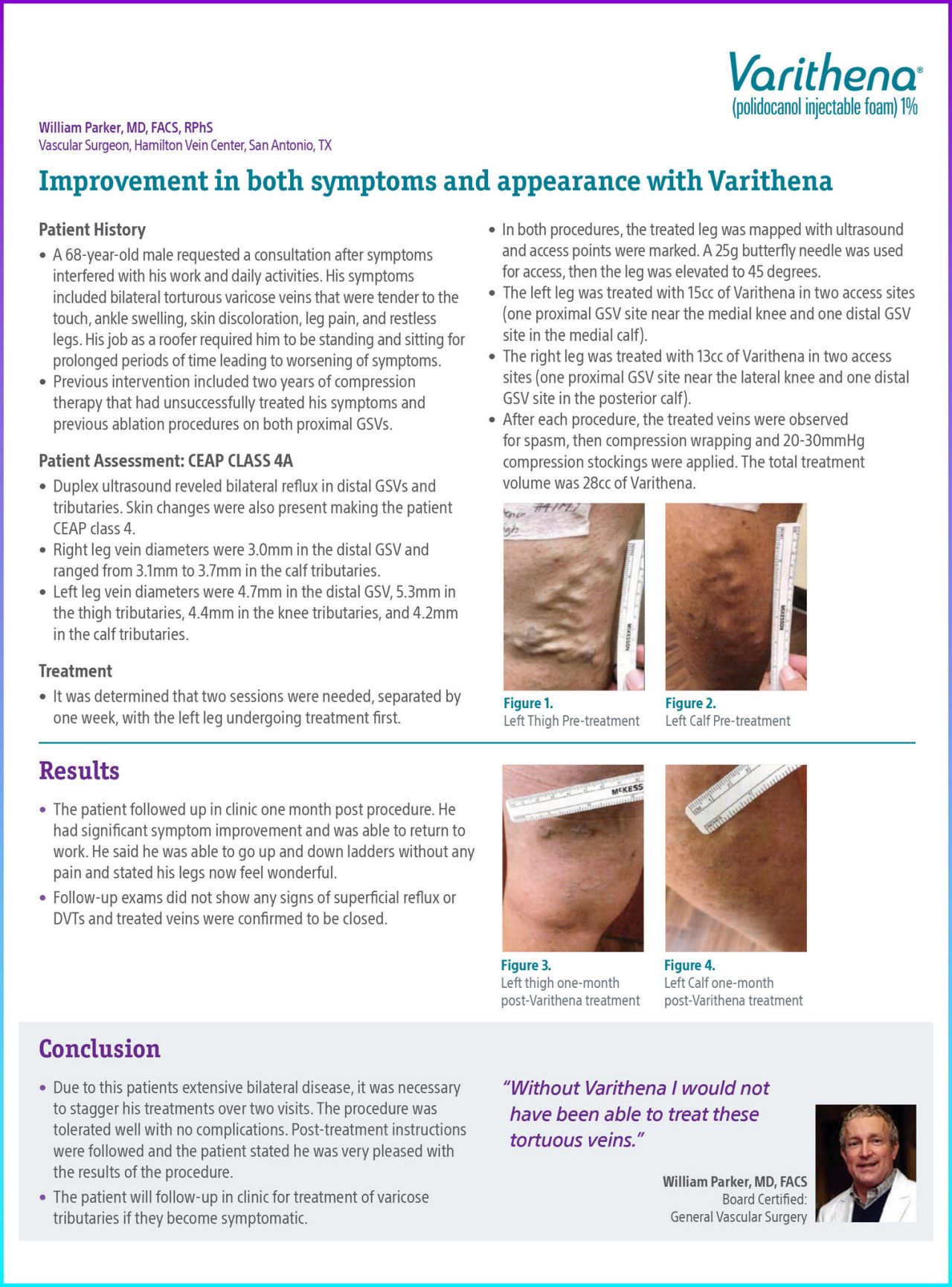
Figure 1. Left thigh pre-treatment.

Figure 2. Left calf pre-treatment.

Figure 3. Left thigh one month post-Varithena treatment.

Figure 4. Left calf one month post-Varithena treatment.