Patient characteristics
A 58-year-old male with C3 disease presented with a long history of persistent symptoms of aching, pain, and heaviness in his right leg.
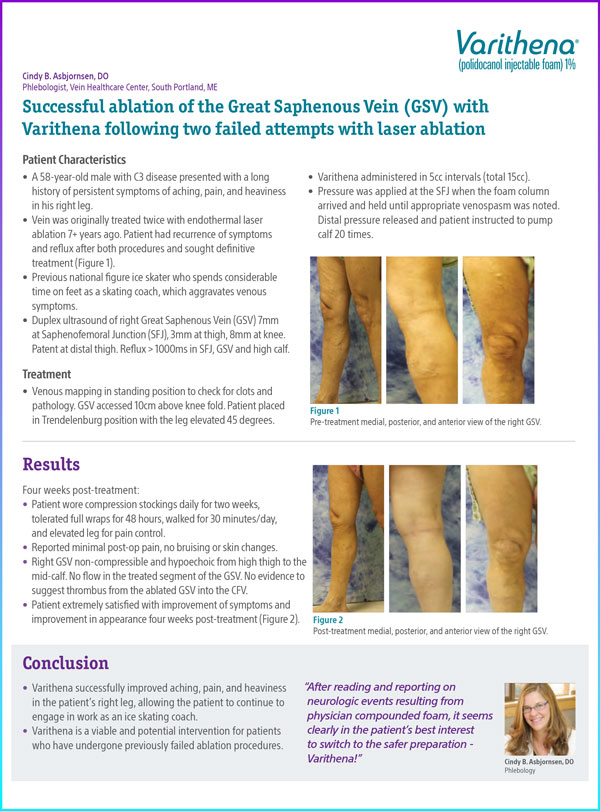
Vein was originally treated twice with endothermal laser ablation 7+ years ago. Patient had recurrence of symptoms and reflux after both procedures and sought definitive treatment (Figure 1).
Previous national figure ice skater who spends considerable time on feet as a skating coach, which aggravates venous symptoms.
Duplex ultrasound of right Great Saphenous Vein (GSV) 7mm at Saphenofemoral Junction (SFJ), 3mm at thigh, 8mm at knee. Patent at distal thigh. Reflux > 1000ms in SFJ, GSV and high calf.
Treatment and results
Venous mapping in standing position to check for clots and pathology. GSV accessed 10cm above knee fold. Patient placed in Trendelenburg position with the leg elevated 45 degrees.
Varithena administered in 5cc intervals (total 15cc).
Pressure was applied at the SFJ when the foam column arrived and held until appropriate venospasm was noted. Distal pressure released and patient instructed to pump calf 20 times.
Four weeks post-treatment:
- Patient wore compression stockings daily for two weeks, tolerated full wraps for 48 hours, walked for 30 minutes/day, and elevated leg for pain control
- Reported minimal post-op pain, no bruising or skin changes
- Right GSV non-compressible and hypoechoic from high thigh to the mid-calf. No flow in the treated segment of the GSV. No evidence to suggest thrombus from the ablated GSV into the CFV
- Patient extremely satisfied with improvement of symptoms and improvement in appearance four weeks post-treatment (Figure 2)
Conclusion
Varithena successfully improved aching, pain, and heaviness in the patient’s right leg, allowing the patient to continue to engage in work as an ice skating coach.
Varithena is a viable and potential intervention for patients who have undergone previously failed ablation procedures.
Documentation

Figure 1. Pre-treatment medial, posterior, and anterior view of the right GSV.

Figure 2. Post-treatment medial, posterior, and anterior view of the right GSV.
* Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.