Patient characteristics
A 68-year-old, otherwise healthy, male with lifelong severe bilateral varicose veins presented to the clinic with pain, heaviness, itching and foot discomfort.
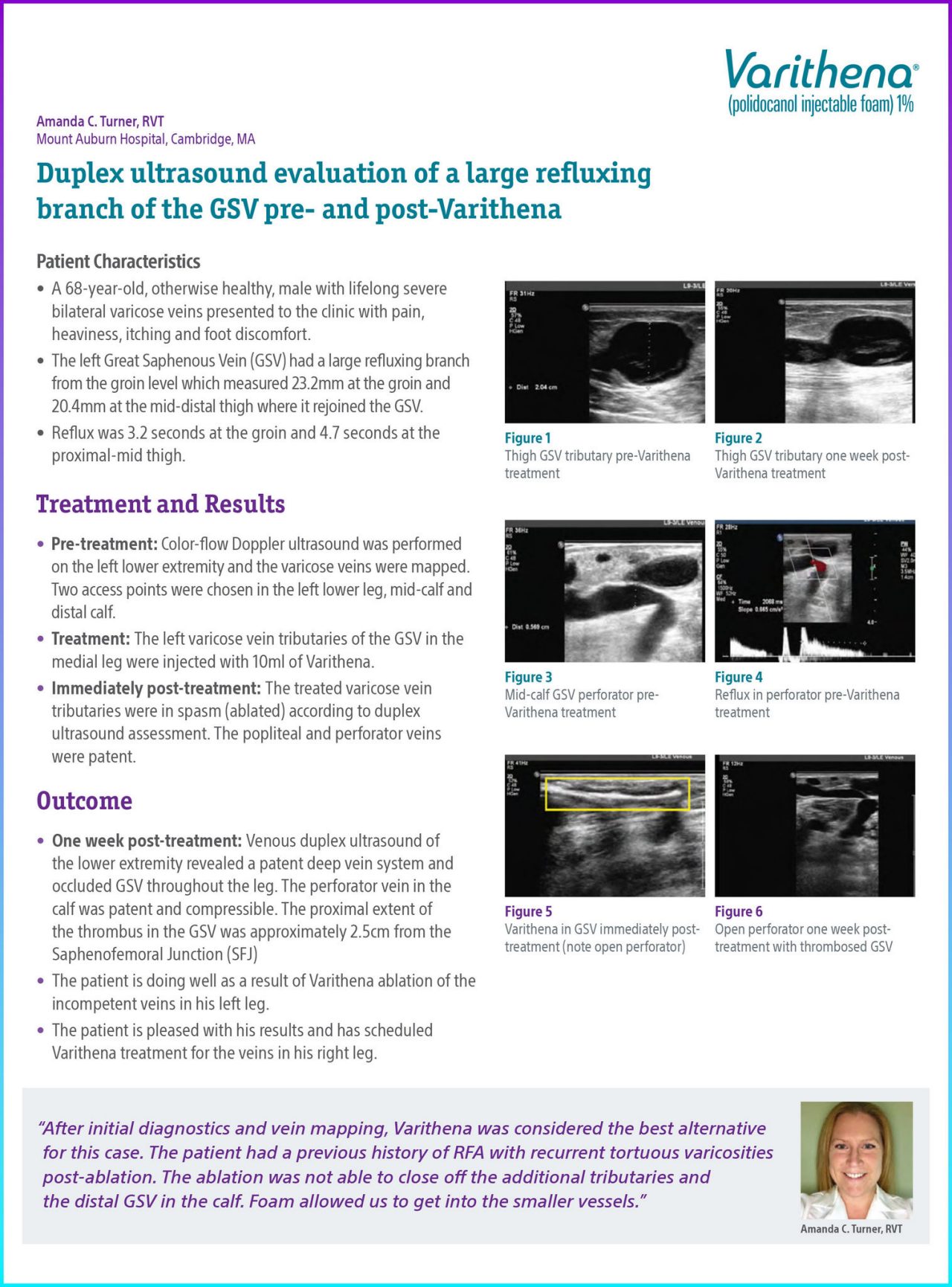
The left Great Saphenous Vein (GSV) had a large refluxing branch from the groin level which measured 23.2mm at the groin and 20.4mm at the mid-distal thigh where it rejoined the GSV.
Reflux was 3.2 seconds at the groin and 4.7 seconds at the proximal-mid thigh.
Treatment and results
Pre-treatment: Color-flow Doppler ultrasound was performed on the left lower extremity and the varicose veins were mapped. Two access points were chosen in the left lower leg, mid-calf and distal calf.
Treatment: The left varicose vein tributaries of the GSV in the medial leg were injected with 10ml of Varithena.
Immediately post-treatment: The treated varicose vein tributaries were in spasm (ablated) according to duplex ultrasound assessment. The popliteal and perforator veins were patent.
Conclusion
One week post-treatment: Venous duplex ultrasound of the lower extremity revealed a patent deep vein system and occluded GSV throughout the leg. The perforator vein in the calf was patent and compressible. The proximal extent of the thrombus in the GSV was approximately 2.5cm from the Saphenofemoral Junction (SFJ).
The patient is doing well as a result of Varithena ablation of the incompetent veins in his left leg.
The patient is pleased with his results and has scheduled Varithena treatment for the veins in his right leg.
Documentation

Figure 1. Thigh GSV Tributary pre-Varithena treatment.

Figure 2. Thigh GSV Tributary one week post- Varithena treatment.

Figure 3. Mid-calf GSV perforator pre-Varithena treatment.

Figure 4. Reflux in perforator pre-Varithena treatment.

Figure 5. Varithena in GSV immediately post-treatment (note open perforator).

Figure 6. Open perforator one week post-treatment with thrombosed GSV.