Count on the treatment that's backed by clinically-proven success.
See the highlights from Varithena clinical studies and real-world outcomes.
VANISH-1 and VANISH-2
Two randomized, multicenter controlled trials demonstrated the efficacy and durability of Varithena treatment in the GSV and led to FDA approval.
Percentage of patients reporting clinically meaningful improvement

Clinically meaningful improvement in symptoms.1
Improvement in symptoms is measured by change in VVSymQ® Score from baseline to Week 8. At Week 8, VVSymQ* scores for patients being treated with Varithena (polidocanol injectable foam) 1% had decreased significantly from baseline.
Clinician-assessed improvement in appearance of varicose veins

Significantly superior improvement in appearance.2
Improvement in appearance is measured by change in IPR-V3 (independent photography review of visible varicose veins) rating from baseline to Week 8. By Week 8, physicians found meaningful improvement in appearance of varicose veins.
*VVSymQ: A patient-reported outcome instrument developed in accordance with FDA guidance. Patients rated a daily duration of 5 varicose vein symptoms: heaviness, achiness, swelling, and throbbing.
Real-world outcomes
Varithena’s record of safety and efficacy isn’t limited to clinical studies. The key findings below are from a collection of real-world studies and peer-reviewed journals. Click the link to view more in-depth summaries.

Varithena polidocanol endovenous microfoam was shown to be generally more stable and durable than physician compounded foams.3

Varithena was shown to be comparable in safety and efficacy to endovenous laser ablation (EVLA) with elimination of reflux in 93.5% and 92.8% of Varithena and EVLA-treated patients, respectively.4

Fewer patients required retreatment if they were treated with both endovenous thermal ablation (ETA) and Varithena, rather than solely ETA.5

A retrospective patient chart review showed complete elimination of venous valvular reflux and vein closure in 94.4% of patients.6
Before & after treatment
These photos show results after one treatment with Varithena.* Additional treatments may be needed, depending on the number and size of veins to be treated.
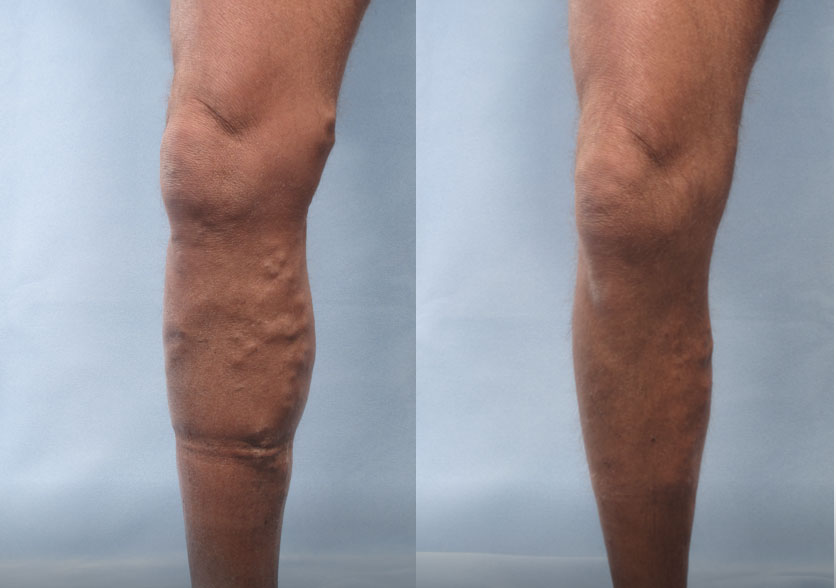
Patient 1:
(Left) Baseline: Moderate
(Right) Week 8: Mild
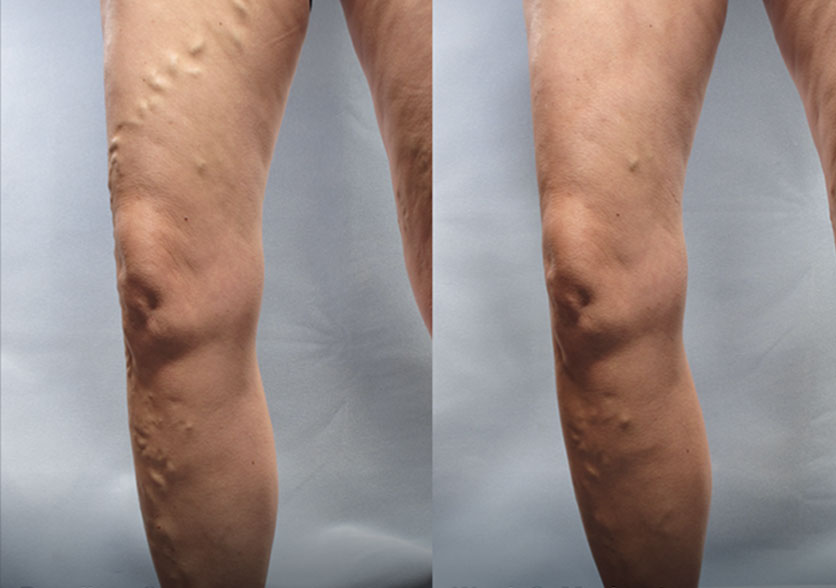
Patient 2:
(Left) Baseline: Severe
(Right) Week 8: Moderate
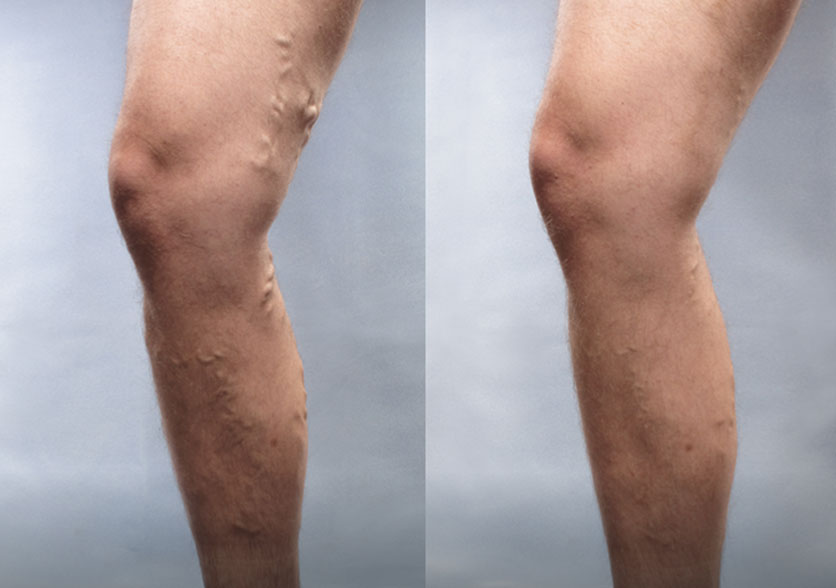
Patient 3:
(Left) Baseline: Moderate
(Right) Week 8: Mild
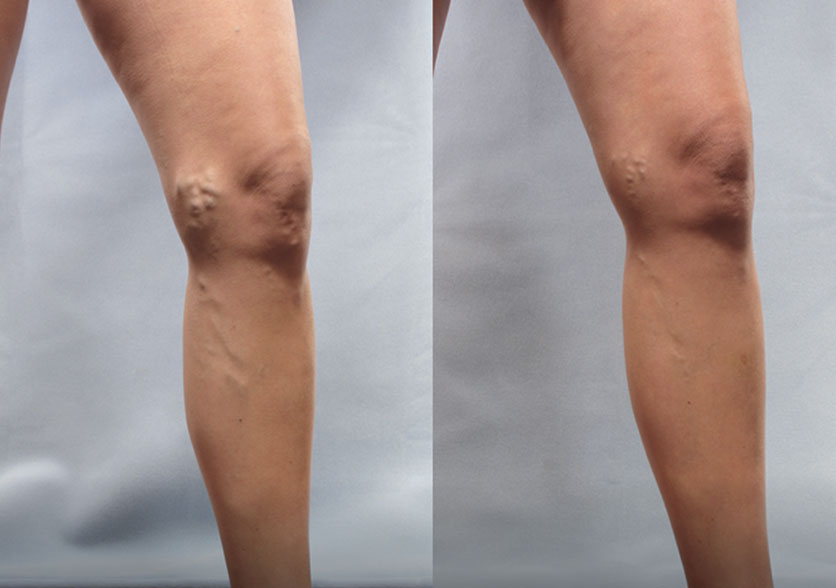
Patient 4:
(Left) Baseline: Moderate
(Right) Week 8: Mild
* All images compare foams within approximately 10 seconds of creation. Photos of physician-compounded foam feature examples of manually created foam made 1:4 with 1% polidocanol solution and room air, Tessari technique. Because of varying conditions and techniques, properties of physician-compounded foams may vary. Photos by RSSL (Reading Scientific Services Ltd, UK).

Case studies
Hear from your peers. Read case study summaries* that detail treatment processes and outcomes and explain why physicians chose Varithena to treat patients’ varicose veins.
* Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.
References
- King JT, O’Byrne M, Vasquez M, Wright D; VANISH-1 Investigator Group. Treatment of Truncal Incompetence and Varicose Veins with a Single Administration of a New Polidocanol Endovenous Microfoam Preparation Improves Symptoms and Appearance. Eur J Vasc Endovasc Surg. 2015 Dec;50(6):784-93. doi: 10.1016/j.ejvs.2015.06.111. Epub 2015 Sep 16. PMID: 26384639.
- Todd KL III, Wright DI, and the VANISH-2 Investigator Group. The VANISH-2 study: a randomized, blinded, multicenter study to evaluate the efficacy and safety of polidocanol endovenous microfoam 0.5% and 1.0% compared with placebo for the treatment of saphenofemoral junction incompetence. Phlebology. 2014;29(9):608-618. doi:10.1177/0268355513497709.
- Carugo D, Ankrett DN, Zhao X, et al. Phlebology: May 2016; 31(4):283-95.
- Deak ST. Treatment of superficial venous insufficiency in a large patient cohort with retrograde administration of ultrasound-guided polidocanol endovenous microfoam versus endovenous laser ablation. J Vasc Surg Venous Lymphat Disord. 2021 Dec 24:S2213-333X(21)00609-0. doi: 10.1016/j.jvsv.2021.11.007. Epub ahead of print. PMID: 34958977.
- Vasquez, Michael. “A multicenter, randomized, placebo-controlled trial of endovenous thermal ablation with or without polidocanol endovenous microfoam treatment in patients with great saphenous vein incompetence and visible varicosities.” Phlebology.2017 May;32(4):272-281. doi: 10.1177/0268355516637300. The author is a consultant for Boston Scientific.
- Deak, Steven T. “Retrograde Administration of Ultrasound-Guided Endovenous Microfoam Chemical Ablation for the Treatment of Superficial Venous Insufficiency.” Journal of Vascular Surgery: Venous and Lymphatic Disorders, vol. 6, no. 4, July 2018, pp. 477–484., doi:https://doi.org/10.1016/j.jvsv.2018.03.015. The author is a consultant for Boston Scientific.
