Patient characteristics
36-year-old mother presented to the clinic with ankle swelling, leg cramps, heaviness and tender varicose veins. She sought treatment years ago but was advised to postpone treatment until she had completed her family. The patient was classified as CEAP 4 upon examination.
Duplex ultrasound assessment revealed reflux involving the right Great Saphenous Vein (GSV) from the Saphenofemoral Junction (SFJ) to the ankle. Peak vein diameter was 8.3mm. Reflux (4.4 seconds) was present in the right common femoral vein (CFV). The deep venous system was patent.
Treatment and results
Varithena treatment was planned for the right GSV tributary that extended medially from the mid-thigh to the ankle.
The patient was prepped, and the skin at the access site was anesthetized with 0.2mL 1% lidocaine. Two access points were used: the tributary just above the ankle and at the knee. For both sites, a 21G micropuncture needle was inserted into the tributary followed by the passage of an 0.018 guidewire and a 4F sheath. The patient was placed in Tredelenburg and 5mL of Varithena was administered at each site for a total treatment volume of 10mL.
Following administration of Varithena, venospasm of the treated segments was documented. Ultrasound images were obtained to confirm that the deep system was patent and that the right common femoral vein and SFJ were compressible.
Conclusion
The patient returned post-treatment and indicated that her edema, heaviness, swelling, tiredness, and cramps had subsided in the treated leg. The brownish discoloration continued to fade and the skin returned to a natural pink color within six weeks.
Due to similar problems in her left leg, and the effectiveness of the procedure, the patient has since undergone treatment in that leg with similar results.
Documentation

Figure 1. Right tributary varicosity prior to Varithena treatment.

Figure 2. Above the knee varicosity prior to treatment.

Figure 3. Above the knee varicosity showing an abnormal, refluxing Doppler signal.

Figure 4. Above the knee varicosity. Pre-treatment reflux.
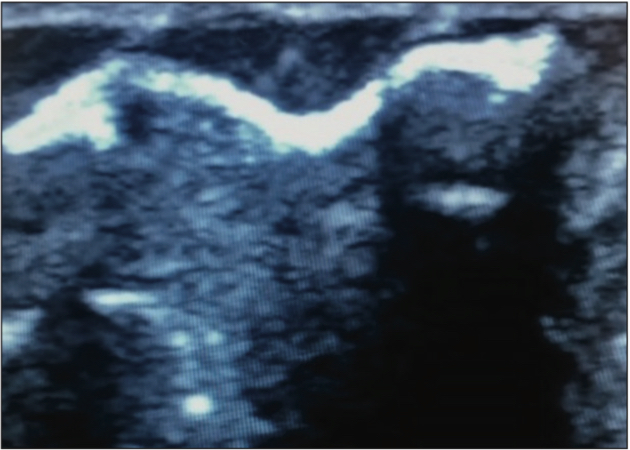
Figure 5. Varithena filling vein during treatment.

Figure 6. Image of vein two weeks post-treatment.
* Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.