Patient characteristics
A 59-year-old male patient presented with a history of varicose veins in bilateral lower extremities since he was a teenager. He has had ulcers in bilateral malleoli of extremities however none active at the time of visit. Patient describes a pain score of 7 on a subjective scale from 0 (no pain) to 10 (most intense pain possible).
Patient’s associated pain and swelling symptoms are exacerbated with sitting and standing and affects his overall ability to work as a golf pro. Symptoms only partially controlled with compression therapy.
With ultrasound evaluation, patient exhibited significant superficial Great Saphenous Vein (GSV) reflux in his right leg as follows:
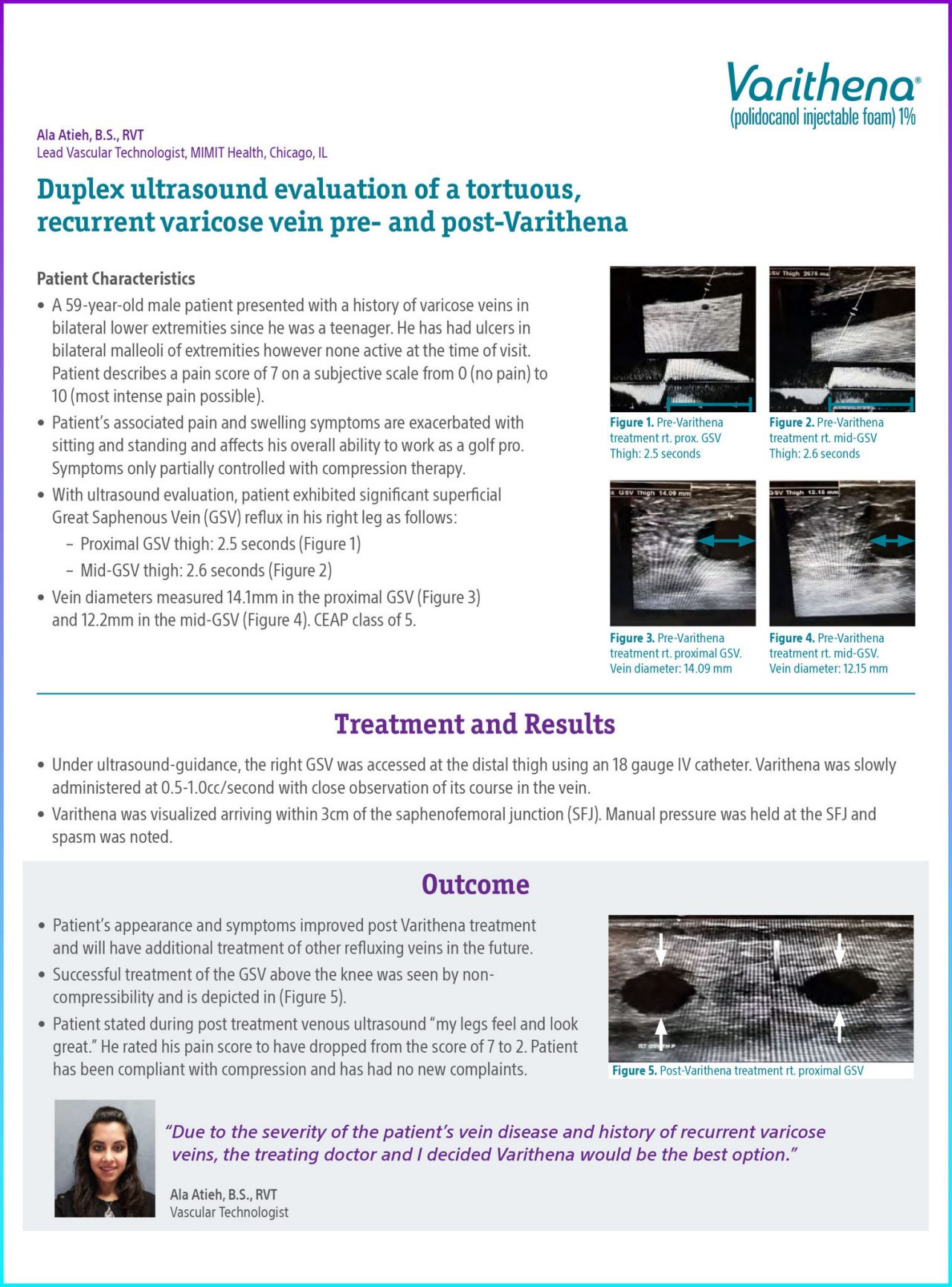
- Proximal GSV thigh: 2.5 seconds (Figure 1)
- Mid-GSV thigh: 2.6 seconds (Figure 2)
Vein diameters measured 14.1mm in the proximal GSV (Figure 3) and 12.2mm in the mid-GSV (Figure 4). CEAP class of 5.
Treatment and results
Under ultrasound-guidance, the right GSV was accessed at the distal thigh using an 18 gauge IV catheter. Varithena was slowly administered at 0.5-1.0cc/second with close observation of its course in the vein.
Varithena was visualized arriving within 3cm of the saphenofemoral junction (SFJ). Manual pressure was held at the SFJ and spasm was noted.
Conclusion
Patient’s appearance and symptoms improved post Varithena treatment and will have additional treatment of other refluxing veins in the future.
Successful treatment of the GSV above the knee was seen by non-compressibility and is depicted in Figure 5.
Patient stated during post treatment venous ultrasound “my legs feel and look great.” He rated his pain score to have dropped from the score of 7 to 2. Patient has been compliant with compression and has had no new complaints.
Documentation

Figure 1. Pre-Varithena Treatment Rt. Prox. GSV Thigh: 2.5 seconds

Figure 2. Pre-Varithena Treatment Rt. Mid-GSV Thigh: 2.6 seconds

Figure 3. Pre-Varithena Treatment Rt. Proximal GSV. Vein diameter: 14.09 mm

Figure 4. Pre-Varithena Treatment Rt. Mid-GSV. Vein diameter: 12.15 mm

Figure 5. Post-Varithena Treatment Rt. Proximal GSV