Patient characteristics
A 52-year-old female presented to the clinic with bilateral leg symptoms. She reported that her most severe symptoms were consistently tired legs that felt heavy. She had a history of bleeding varicosities in the past.
As a housekeeper, the prolonged standing for her occupation contributed to a worsening of symptoms and limited her ability to work.
Despite the use of 20-30mmHg prescription compression stockings, she only had partial relief of her symptoms and they did not stay in place during the workday.
Patient Work-up: CEAP Class 3, VCSS 16
- The patient had bilateral pitting edema from her distal thigh to her mid-calf, as well as bulging varicosities on her right anterior distal thigh to mid-calf compartment
- Reflux on ultrasound measured >1-3 seconds from the mid-thigh through the calf in both legs. Vein diameters ranged from 3.8-6.0mm.
Treatment and results
Varithena was chosen for this patient because thermal and tumescent options presented a risk of neuropathy, especially in her distal segments.
5cc of Varithena was administered to both the distal right thigh and calf Great Saphenous Vein (GSV) segments.
The patient returned at three and six months post-treatment for follow-ups. Changes in leg circumference reflected reductions in edema and patient symptoms.
| Segment | Initial Presentation | 6 Months |
|---|---|---|
| Distal Thigh | 65 cm | 54 cm |
| Proximal Calf | 51 cm | 44 cm |
| Mid-Calf | 48 cm | 37 cm |
| Distal Calf | 27 cm | 26 cm |
Conclusion
The patient was not only pleased with the reduction in her symptoms post-treatment, she was able to work a full day with minimal pain and discomfort. She is pleased with the results and its impact on her work and home life.
Documentation

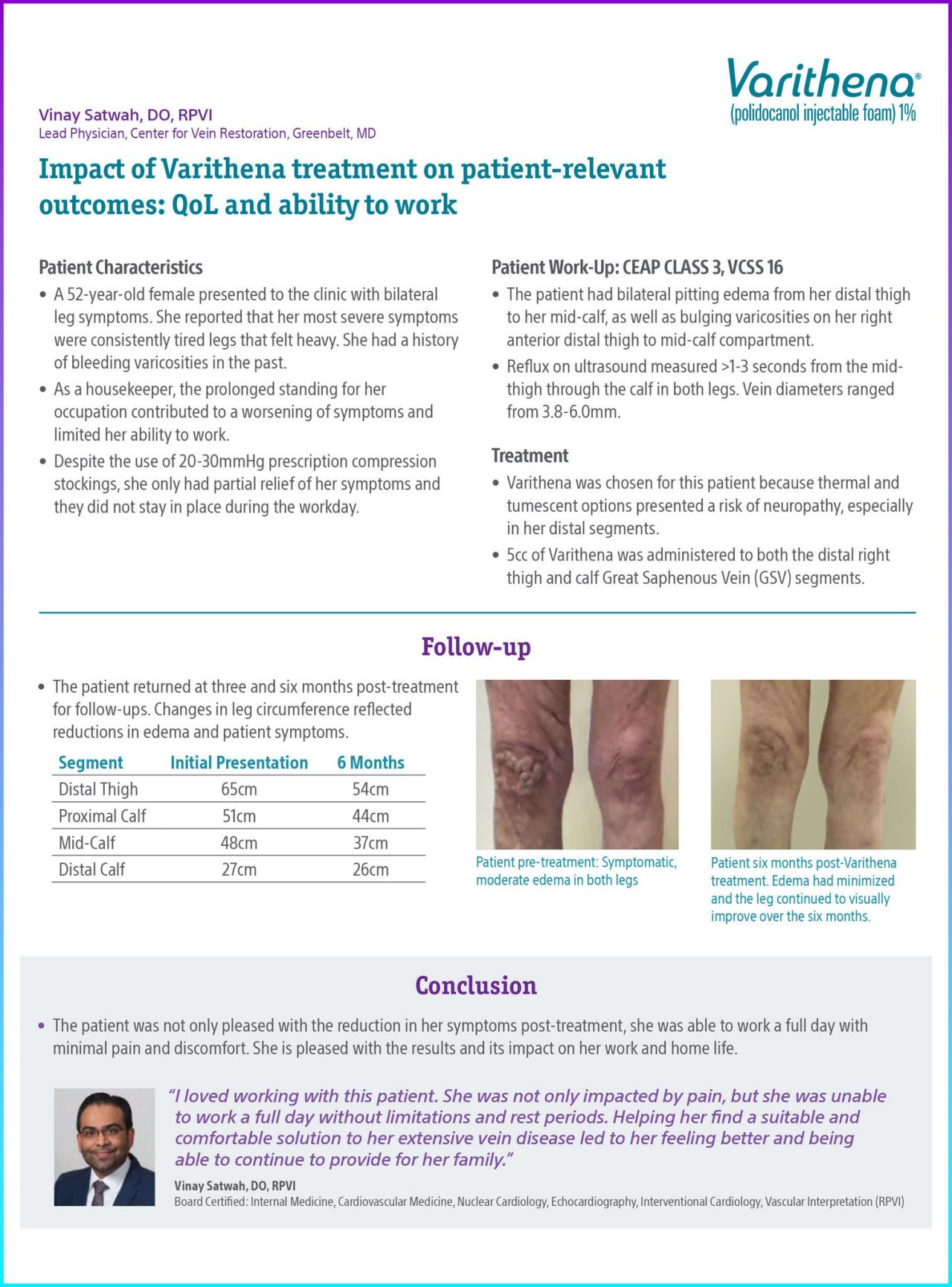
Figure 1. Patient pre-treatment: Symptomatic, moderate edema in both legs.

Figure 2. Patient six months post-Varithena treatment. Edema had minimized and the leg continued to visually improve over the six months.