Study design
VANISH-1 was a randomized, blinded, multicenter parallel group study designed to evaluate the efficacy and safety of 3 concentrations of polidocanol injectable foam, Varithena 0.5%, 1%, and 2%, compared with a placebo. A subtherapeutic dose of Varithena 0.125% was included as a control for blinding. The study population (n=279) consisted of patients who had saphenofemoral junction (SFJ) incompetence due to reflux of the great saphenous vein (GSV) or major accessory veins as determined by duplex ultrasound and superficial venous disease manifested by both symptoms and visible varicosities. The primary efficacy endpoint was patient-reported venous symptom improvement measured by change from baseline to Week 8 in 7-day average Varicose Veins Symptoms Questionnaire (VVSymQ®) score. Co-secondary efficacy endpoints assessed improvement in appearance of visible varicose veins from baseline to Week 8 as measured by scores of the Independent Photography Review–Visible Varicose Veins (IPR-V3) as well as the Patient Self-assessment of Visible Varicose Veins (PA-V3). The 3 tertiary endpoints were ultrasound response, change in Venous Clinical Severity Score (VCSS), and the modified Venous Insufficiency Epidemiological and Economic Study–Quality of Life/Symptoms (VEINES-QOL/Sym) score.
Polidocanol endovenous microfoam 1.0% is FDA approved as Varithena (polidocanol injectable foam) 1%.
Indications
Varithena (polidocanol injectable foam) 1% is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
VANISH-1: Efficacy results
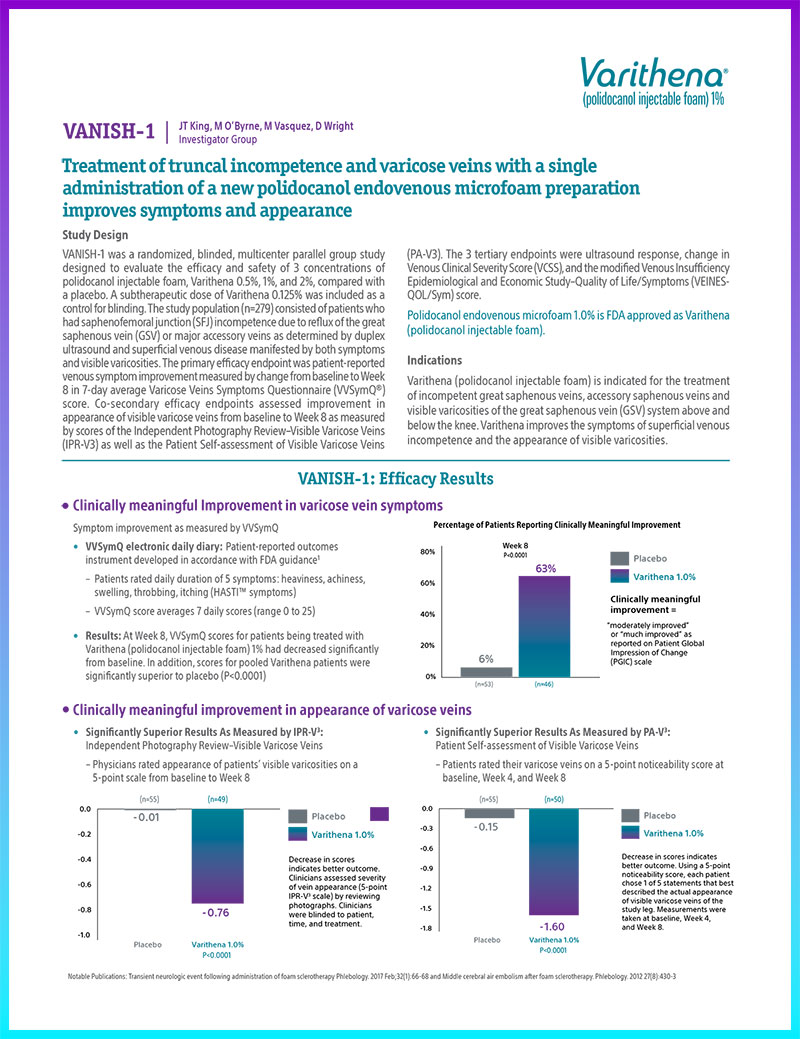
Clinically meaningful improvement in varicose vein symptoms
Symptom improvement as measured by VVSymQ
- VVSymQ electronic daily diary: Patient-reported outcomes instrument developed in accordance with FDA guidance1
- Patients rated daily duration of 5 symptoms: heaviness, achiness, swelling, throbbing, itching (HASTI™ symptoms)
- VVSymQ score averages 7 daily scores (range 0 to 25)
- Results: At Week 8, VVSymQ scores for patients being treated with Varithena (polidocanol injectable foam) 1% had decreased significantly from baseline. In addition, scores for pooled Varithena patients were significantly superior to placebo (P<0.0001)
Percent of patients reporting clinically meaningful improvement

Clinically meaningful improvement in appearance of varicose veins
Significantly Superior Results As Measured by IPR-V3:
Independent Photography Review–Visible Varicose Veins
- Physicians rated appearance of patients’ visible varicosities on a 5-point scale from baseline to Week 8

Significantly Superior Results As Measured by PA-V3:
Patient Self-assessment of Visible Varicose Veins
- Patients rated their varicose veins on a 5-point noticeability score at baseline, Week 4, and Week 8

Notable Publications: Transient neurologic event following administration of foam sclerotherapy Phlebology. 2017 Feb;32(1):66-68 and Middle cerebral air embolism after foam sclerotherapy. Phlebology. 2012 27(8):430-3
Tertiary endpoints
Improvement as measured through duplex ultrasound response, VCSS, and VEINES-QOL/Sym
- Duplex ultrasound response
- The duplex response rate for patients treated with Varithena (polidocanol injectable foam) 1% was 80.4%, demonstrating a significant superiority to the rate of those patients treated with Varithena 0.125%
- VCSS and VEINES-QOL/Sym
- For patients treated with Varithena 1%, improvements in revised VCSS and VEINES-QOL/Sym scores at Week 8 were statistically superior to changes observed in the placebo group (P≤0.0001 in all cases)
- Additionally, mean changes in revised VCSS and VEINES-QOL/Sym scores at Week 8 for patients in the pooled Varithena 0.5%, 1%, and 2% group were statistically superior to changes observed in the Varithena 0.125% group (P=0.0038 and P=0.0073)
VANISH-1: Safety profile
In this trial, there were no serious adverse events (AEs) and no occurrences of pulmonary embolism (PE)
- In the 174 patients treated with any dose of Varithena that experienced AEs, 42% were mild while 18% were moderate. Severe AEs were seen in 12 patients
- Most common AEs: pain in extremity, superficial thrombophlebitis, infusion site thrombosis, injection site hematoma
VANISH-1: Conclusions
Varithena:
- Demonstrated significant and clinically meaningful improvement in both the symptoms and the appearance of varicose veins in patients with an incompetent GSV and/or accessory saphenous veins and visible varicosities
- Is a minimally invasive, generally safe, non-surgical procedure
- Is associated with mostly mild or moderate adverse events with most resolved without sequel AE
- The most common adverse events observed were pain in extremity, superficial thrombophlebitis, infusion site thrombosis, and injection site hematoma
References
1. US Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Washington, DC: US Department of Health and Human Services; 2009