Patient characteristics
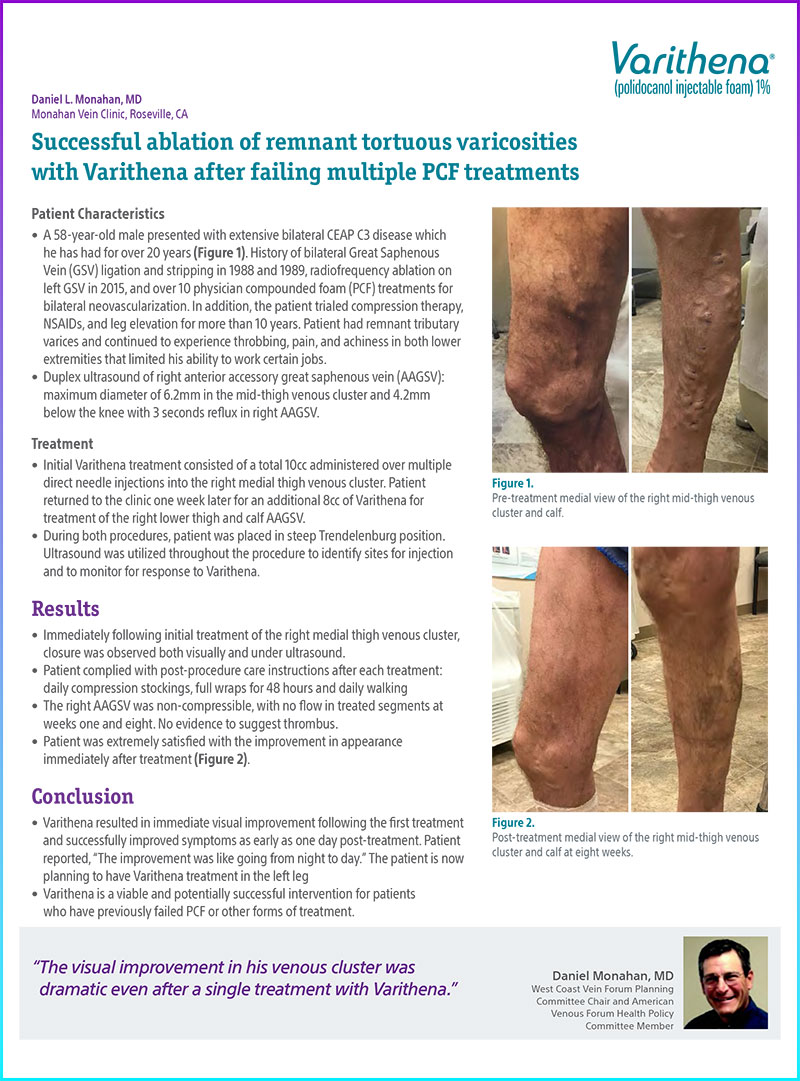
A 58-year-old male presented with extensive bilateral CEAP C3 disease which he has had for over 20 years (Figure 1). History of bilateral Great Saphenous Vein (GSV) ligation and stripping in 1988 and 1989, radiofrequency ablation on left GSV in 2015, and over 10 physician compounded foam (PCF) treatments for bilateral neovascularization. In addition, the patient trialed compression therapy, NSAIDs, and leg elevation for more than 10 years. Patient had remnant tributary varices and continued to experience throbbing, pain, and achiness in both lower extremities that limited his ability to work certain jobs.
Duplex ultrasound of right anterior accessory great saphenous vein (AAGSV): maximum diameter of 6.2mm in the mid-thigh venous cluster and 4.2mm below the knee with 3 seconds reflux in right AAGSV.
Treatment and results
Initial Varithena treatment consisted of a total 10cc administered over multiple direct needle injections into the right medial thigh venous cluster. Patient returned to the clinic one week later for an additional 8cc of Varithena for treatment of the right lower thigh and calf AAGSV.
During both procedures, patient was placed in steep Trendelenburg position. Ultrasound was utilized throughout the procedure to identify sites for injection and to monitor for response to Varithena.
Immediately following initial treatment of the right medial thigh venous cluster, closure was observed both visually and under ultrasound.
Patient complied with post-procedure care instructions after each treatment: daily compression stockings, full wraps for 48 hours and daily walking.
The right AAGSV was non-compressible, with no flow in treated segments at weeks one and eight. No evidence to suggest thrombus.
Patient was extremely satisfied with the improvement in appearance immediately after treatment (Figure 2).
Conclusion
Varithena resulted in immediate visual improvement following the first treatment and successfully improved symptoms as early as one day post-treatment. Patient reported, “The improvement was like going from night to day.” The patient is now planning to have Varithena treatment in the left leg.
Varithena is a viable and potentially successful intervention for patients who have previously failed PCF or other forms of treatment.
Documentation

Figure 1. Pre-treatment medial view of the right mid-thigh venous cluster and calf.

Figure 2. Post-treatment medial view of the right mid-thigh venous cluster and calf at eight weeks.
* Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.