Patient characteristics
A 39-year-old male presented to the clinic with bilateral lower extremity C6 venous disease. The patient had recurrent venous leg ulcerations (VLUs) and recently had two episodes of severe bleeding from a right lower extremity varicosity at the site of ulceration, one requiring a visit to the emergency room.
While the ulcers have been present for five weeks, the patient has an 8-year history of progressive venous disease in both his legs.
The patient has used analgesics and compression hose to help with his chronic symptoms of leg pain, aching, itching, burning and ankle/leg swelling.
Patient work-up: CEAP class 6
- Vein diameters in the left leg ranged from 3.5mm in the SSV, to 11.6mm in the GSV, to 14.5 at the CFV. In the right leg, diameters were 4.9mm, 14.2mm, and 17.2mm, in the SSV, GSV, and CFV, respectively
- The patient did not have a history of prior vein treatment
Treatment and results
Due to the extensive bilateral disease above and below the knee, laser ablation and Varithena were used. Varithena was used in all tortuous segments of the GSV. The patient required two treatments of Varithena for each leg.
For each treatment, access was gained using a 21-gauge butterfly needle. A direct access approach was chosen because the patient had many tortuous segments.
The leg was raised to decrease GSV diameter, reducing the amount of Varithena needed to treat his large veins.
In the left leg, six injections were used in the below-knee GSV to deliver 12cc and 6cc of Varithena on separate occasions. In the right leg, five injections were used to deliver 14cc and 7cc to above- and below-knee GSV segments on separate occasions.
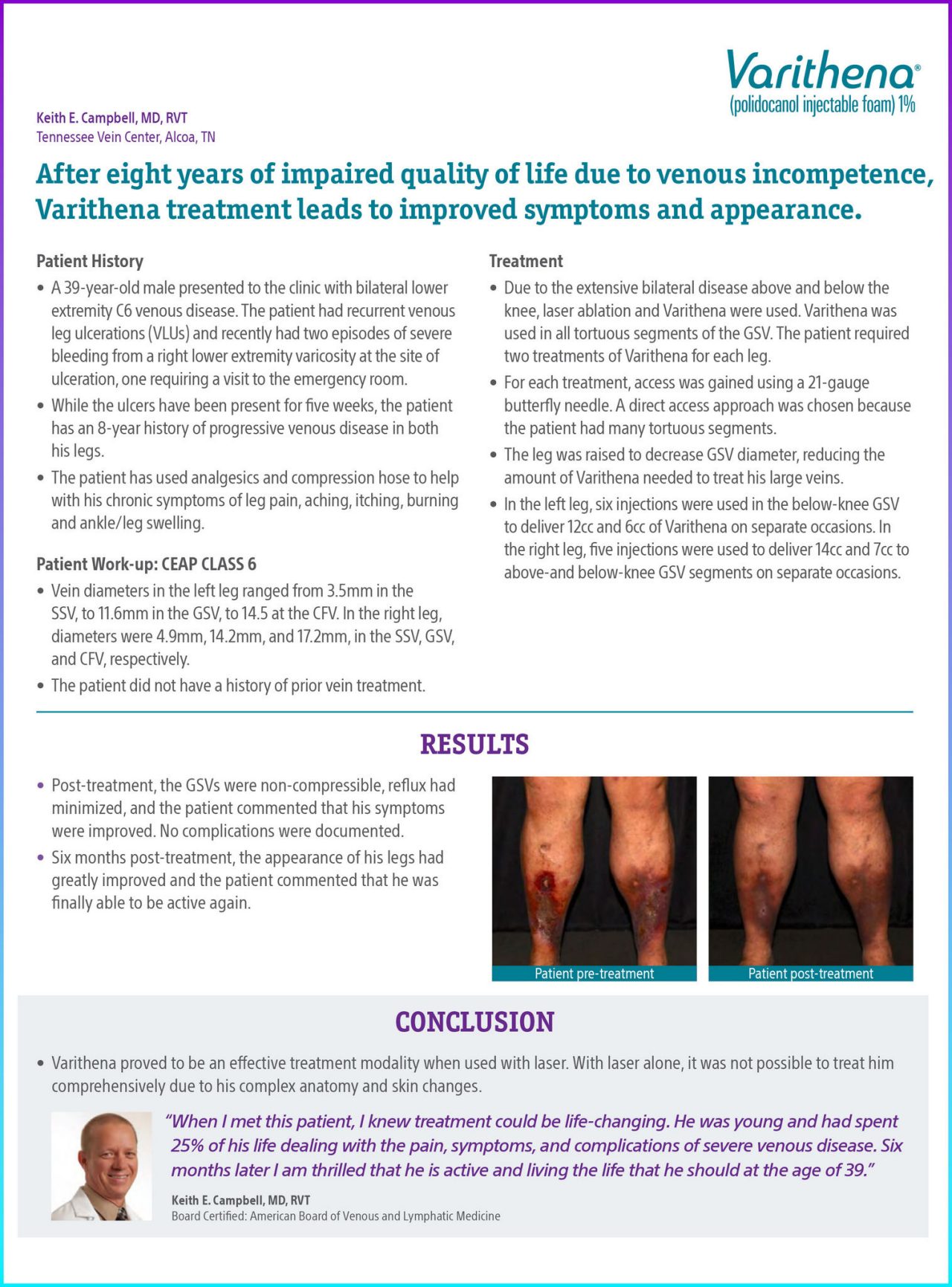
Post-treatment, the GSVs were non-compressible, reflux had minimized, and the patient commented that his symptoms were improved. No complications were documented.
Six months post-treatment, the appearance of his legs had greatly improved and the patient commented that he was finally able to be active again.
Conclusion
Varithena proved to be an effective treatment modality when used with laser. With laser alone, it was not possible to treat him comprehensively due to his complex anatomy and skin changes.
Documentation

Figure 1. Patient Pre-treatment.

Figure 2. Varithena was used in all tortuous segments of the GSV above and below the knee. The patient required two treatments of Varithena for each leg. Laser ablation was also used in straight segments of the proximal GSV.