Dr. Carugo's 2016 study
Carugo D, Ankrett DN, Zhao X, et al.
Phlebology: May 2016; 31(4):283-95.
Objective
The objective of this study was to compare physician-compounded foams (PCFs) with polidocanol endovenous microfoam (PEM).
Introduction and Methods Unlike PCFs, PEM is generated by a proprietary device that produces consistent, pharmaceutical-grade low-nitrogen foam (<0.8%), O2 :CO2 (65:35). For preparation of PCFs, 1% aqueous buffered polidocanol solution was used. Two different foam densities were used when creating PCFs: a liquid:gas ratio of 1:7 was used as a direct comparison to PEM while a 1:4 ratio was used to represent common formulations. Four key measurements were used to compare and contrast PEM and PCFs. These helped to highlight key characteristics of each foam and what made them effective. The four measurements were bubble size and size distribution, foam drainage time (FDT), foam half time (FHT), and degradation rate/dwell time (DR/DT). Several different kinds of equipment were used to generate these measurements.
- Glass-plate method: analyzed bubble size and bubble size distribution
- Sympatec QICPIC image analysis sensor: analyzed bubble size and bubble size distribution
- Turbiscan™ LAB apparatus: measured foam drainage time and foam half time, both methods of measuring foam stability
- Biomimetic vein model: measured foam stability using foam dwell time and degradation rate
Key Results
Four key measurements were used to compare PEM and PCFs:
Bubble size distribution
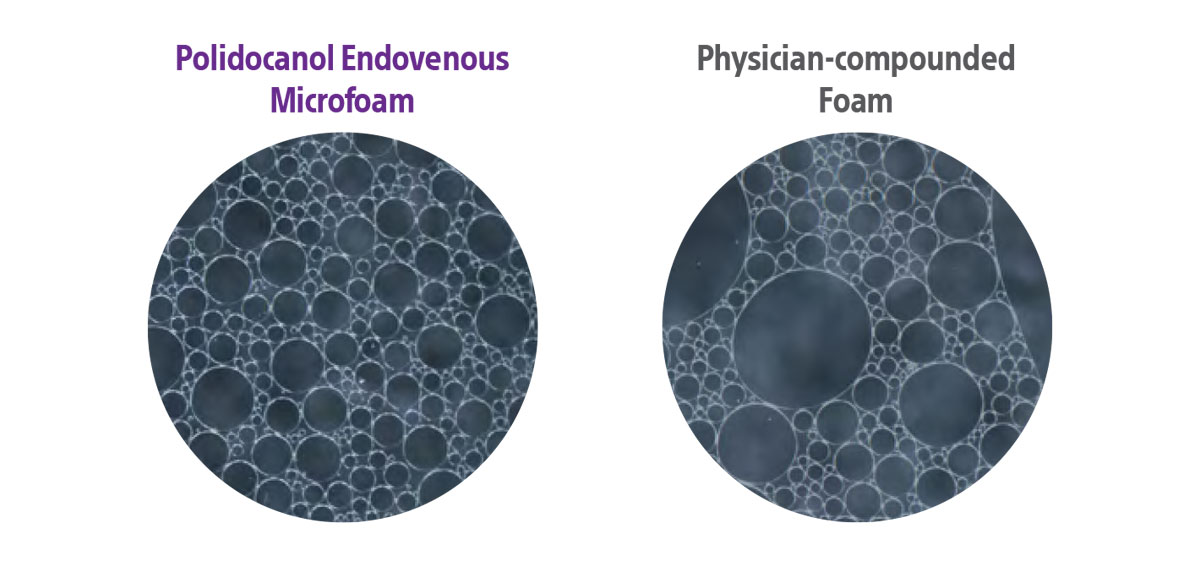
- Bubble size distribution of PEM was narrower when compared with PCFs
- The same gas mixture and liquid:gas ratio were used with either the double syringe system (DSS) or Tessari method
Foam drainage time
- The FDT of PEM was measured against two types of PCF
- Liquid:gas ratio of 1:4 and 1:7
- PEM had a significantly longer FDT than PCFs prepared with either method at either liquid:gas ratio
- The only exception was the PCF prepared with room air using the DSS method
Examples of PEM bubble size compared with PCF bubble size
(photos not directly connected to this study).*
* Carugo D, Ankrett DN, Zhao X, Zhang X, Hill M, O'Byrne V, Hoad J, Arif M, Wright DD, Lewis AL. Benefits of polidocanol endovenous microfoam (Varithena®) compared with physician-compounded foams. Phlebology. 2016 May;31(4):283-95. doi: 10.1177/0268355515589063. Epub 2015 Jun 1. PMID: 26036246; PMCID: PMC4838175.
Foam half time
- The foam half time of PEM and PCFs generated through the DSS and Tessari methods were compared using the Turbiscan™ LAB apparatus
- Liquid:gas ratio of 1:4 and 1:7
- PEM displayed a longer FHT when compared to all PCFs that contain CO2 , demonstrating that PEM was generally more stable than PCFs
Degradation rate and dwell time
- A biomimetic vein model was used to assess the ability of foam to displace a blood substitute
- PEM was shown to have a statistically lower DR when compared with CO2 - containing PCFs prepared with either the DSS or Tessari method and liquid:gas ratios of 1:4 or 1:7
- PEM had the longest calculated DT when compared to PCFs using room air and CO2 generated through either the DSS or Tessari method
- The DT of PEM was shown to be nearly twice that of PCFs that used room air and approximately 8 times that of PCFs that used equivalent mixtures
- The DT of PEM was shown to be nearly twice that of PCFs that used room air and approximately 8 times that of PCFs that used equivalent mixtures
Conclusions
PEM was shown to be generally more stable and durable than PCFs:
Smaller and more consistent bubble size than PCFs
It has been shown that foams with smaller and more uniform bubble size possess a lower DR, which indicates a more cohesive and stable foam. This helps to ensure better contact with the endothelium of the vessel wall upon injection.
Longer foam drainage time than PCFs for greater durability
The ideal foam should be durable enough to allow injection before separating into its gas and liquid components, yet shortlived enough to break down once injected. These qualities were demonstrated for PEM using measures of FDT, FHT, and DR/DT.
Absorbability for potentially enhanced safety
The absence of nitrogen and the efficacy of stable small bubble foam may provide PEM with the safety benefit of absorbability.
“The [polidocanol endovenous microfoam] PEM made with O2:CO2, low nitrogen-gas composition and proprietary foam generation device results in better overall performance than [physician-compounded foam] PCF in a variety of tests, without the associated risk of high-nitrogen [room air] RA bubbles.”
— Carugo D, et al. Phlebology: 2015.
References
Carugo D, Ankrett DN, Zhao X, Zhang X, Hill M, O'Byrne V, Hoad J, Arif M, Wright DD, Lewis AL. Benefits of polidocanol endovenous microfoam (Varithena®) compared with physician-compounded foams. Phlebology. 2016 May;31(4):283-95. doi: 10.1177/0268355515589063. Epub 2015 Jun 1. PMID: 26036246; PMCID: PMC4838175.
Indications
Varithena (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
Important Safety Information
The use of Varithena is contraindicated in patients with known allergy to polidocanol and those with acute thromboembolic disease. Severe allergic reactions have been reported following administration of liquid polidocanol, including anaphylactic reactions, some of them fatal. Observe patients for at least 10 minutes following injection and be prepared to treat anaphylaxis appropriately. Intra-arterial injection or extravasation of polidocanol can cause severe necrosis, ischemia or gangrene. Patients with underlying arterial disease may be at increased risk for tissue ischemia. If intra- arterial injection of polidocanol occurs, consult a vascular surgeon immediately. Varithena can cause venous thrombosis. Follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization, or pregnancy are at increased risk for developing thrombosis. The most common adverse events observed were pain/discomfort in extremity, retained coagulum, injection site hematoma or pain, common femoral vein thrombus extension, superficial thrombophlebitis, and deep vein thrombosis. Physicians administering Varithena must be experienced with venous procedures, possess a detailed working knowledge of the use of the duplex ultrasound in venous disease and be trained in the administration of Varithena.
For Full Prescribing Information visit Varithena.com
Varithena™ is a registered trademark of Boston Scientific. All other trademarks are property of their respective owners.
PI-1263705-AA