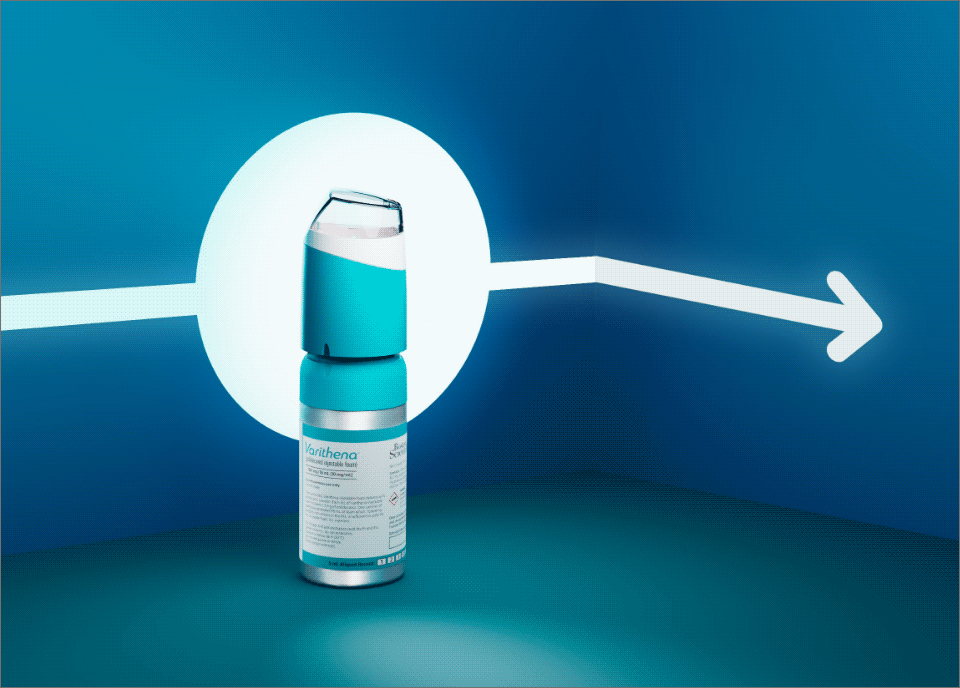
One thing can be a meaningful addition for your patients and your practice.
Varithena goes where thermal can’t, to treat varicose veins caused by venous insufficiency of the great saphenous vein (GSV) system and other truncal veins.
The only FDA-approved microfoam comes pre-formulated to treat all vein shapes and a wide variety of sizes, above and below the knee. Clinically proven, Varithena produces reliable results—time after time.
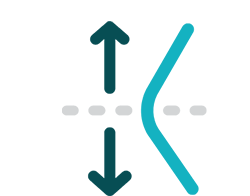
Treat above and below the knee
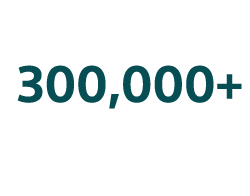
Treatments performed1

Only 1 to 2 needle sticks per treatment
The data all adds up.
See the clinical trials, case studies, and peer-reviewed publications that demonstrate Varithena’s efficacy and versatility.
One exceptional patient experience leads to another.
See the effect of getting patients back to their normal life, the same day. With a procedure time of less than one hour, almost no pain, and little to no downtime for your patients, Varithena treats efficiently and comfortably.
Ready when you are.
Varithena is ready for almost any vein in almost any patient. With a streamlined prep and procedure, you and your staff can be confident in its consistency.
Order
Varithena
Add to your practice with this versatile, efficient, and consistent varicose vein treatment. Order directly from Boston Scientific.
Become Varithena certified
Join the 2,000+ certified physicians improving patients’ lives with Varithena. We’re here to help you, and becoming certified is easy.

Contact a sales representative
Have your local Varithena
sales representative
contact you.

Join the physician
finder
Patients want to know who’s performing Varithena procedures. Get noticed by potential patients.
References
- Data on file
Indications
Varithena® (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena® improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
Important Safety Information
The use of Varithena® is contraindicated in patients with known allergy to polidocanol and those with acute thromboembolic disease. Severe allergic reactions have been reported following administration of liquid polidocanol, including anaphylactic reactions, some of them fatal. Observe patients for at least 10 minutes following injection and be prepared to treat anaphylaxis appropriately. Intra-arterial injection or extravasation of polidocanol can cause severe necrosis, ischemia or gangrene. Patients with underlying arterial disease may be at increased risk for tissue ischemia. If intra-arterial injection of polidocanol occurs, consult a vascular surgeon immediately.Varithena® can cause venous thrombosis. Follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization, or pregnancy are at increased risk for developing thrombosis. The most common adverse events observed were pain/discomfort in extremity, retained coagulum, injection site hematoma or pain, common femoral vein thrombus extension, superficial thrombophlebitis, and deep vein thrombosis.Physicians administering Varithena® must be experienced with venous procedures, possess a detailed working knowledge of the use of the duplex ultrasound in venous disease and be trained in the administration of Varithena®.
See Full Prescribing Information for Varithena®
Varithena™ is a registered trademark of Boston Scientific. All other trademarks are property of their respective owners.
PI-1263705-AA